Advertisements
Ratings
RRB (Railways) Paper Pattern, Syllabus & Books
Chemical & Metallurgical Assistant – (CMA)
RRB 2020 Syllabus – CMA – Here in below table we are listing few topics for each section which were asked in previous examination of RRB Chemical & Metallurgical Assistant (CMA) :
Stage-I
| (1) | Knowledge of Current affairs |
| (2) | Indian geography |
| (3) | culture and history of India including freedom struggle |
| (4) | Indian Polity and constitution |
| (5) | Indian Economy |
| (6) | Environmental issues concerning India and the World |
| (7) | Sports |
| (8) | General scientific and technological developments |
| (1) | Physics |
| (2) | Chemistry |
| (3) | Life Sciences |
General Intelligence & Reasoning
| (1) | Analogies |
| (2) | Alphabetical and Number Series |
| (3) | Coding and Decoding |
| (4) | Mathematical operations |
| (5) | Relationships |
| (6) | Syllogism |
| (7) | Jumbling |
| (8) | Venn Diagram |
| (9) | Data Interpretation and Sufficiency |
| (10) | Conclusions and Decision Making |
| (11) | Similarities and Differences |
| (12) | Analytical reasoning |
| (13) | Classification |
| (14) | Directions |
| (15) | Statement – Arguments and Assumptions |
| (1) | Number systems |
| (2) | BODMAS |
| (3) | Decimals |
| (4) | Fractions |
| (5) | LCM and HCF |
| (6) | Ratio and Proportion |
| (7) | Percentages |
| (8) | Mensuration |
| (9) | Time and Work |
| (10) | Time and Distance |
| (11) | Simple and Compound Interest |
| (12) | Profit and Loss |
| (13) | Algebra |
| (14) | Geometry |
| (15) | Trigonometry |
| (16) | Elementary Statistics |
| (17) | Square Root |
| (18) | Age Calculations |
| (19) | Calendar & Clock |
| (20) | Pipes & Cistern |
Stage-II
| (1) | Knowledge of Current affairs |
| (2) | Indian geography |
| (3) | culture and history of India including freedom struggle |
| (4) | Indian Polity and constitution |
| (5) | Indian Economy |
| (6) | Environmental issues concerning India and the World |
| (7) | Sports |
| (8) | General scientific and technological developments |
Basics of Computers and Applications
| (1) | Architecture of Computers |
| (2) | input and Output devices |
| (3) | Storage devices |
| (4) | Networking |
| (5) | Operating System like Windows, Unix, Linux |
| (6) | MS Office; Various data representation |
| (7) | Internet and Email |
| (8) | Websites & Web Browsers |
| (9) | Computer Virus |
| (1) | Physics |
| (2) | Chemistry |
| (3) | Life Sciences |
Basics of Environment and Pollution Control
| (1) | Basics of Environment |
| (2) | Adverse effect of environmental pollution and control strategies |
| (3) | Air, water and Noise pollution, their effect and control |
| (4) | Waste Management |
| (5) | Global warming |
| (6) | Acid rain |
| (7) | Ozone depletion |
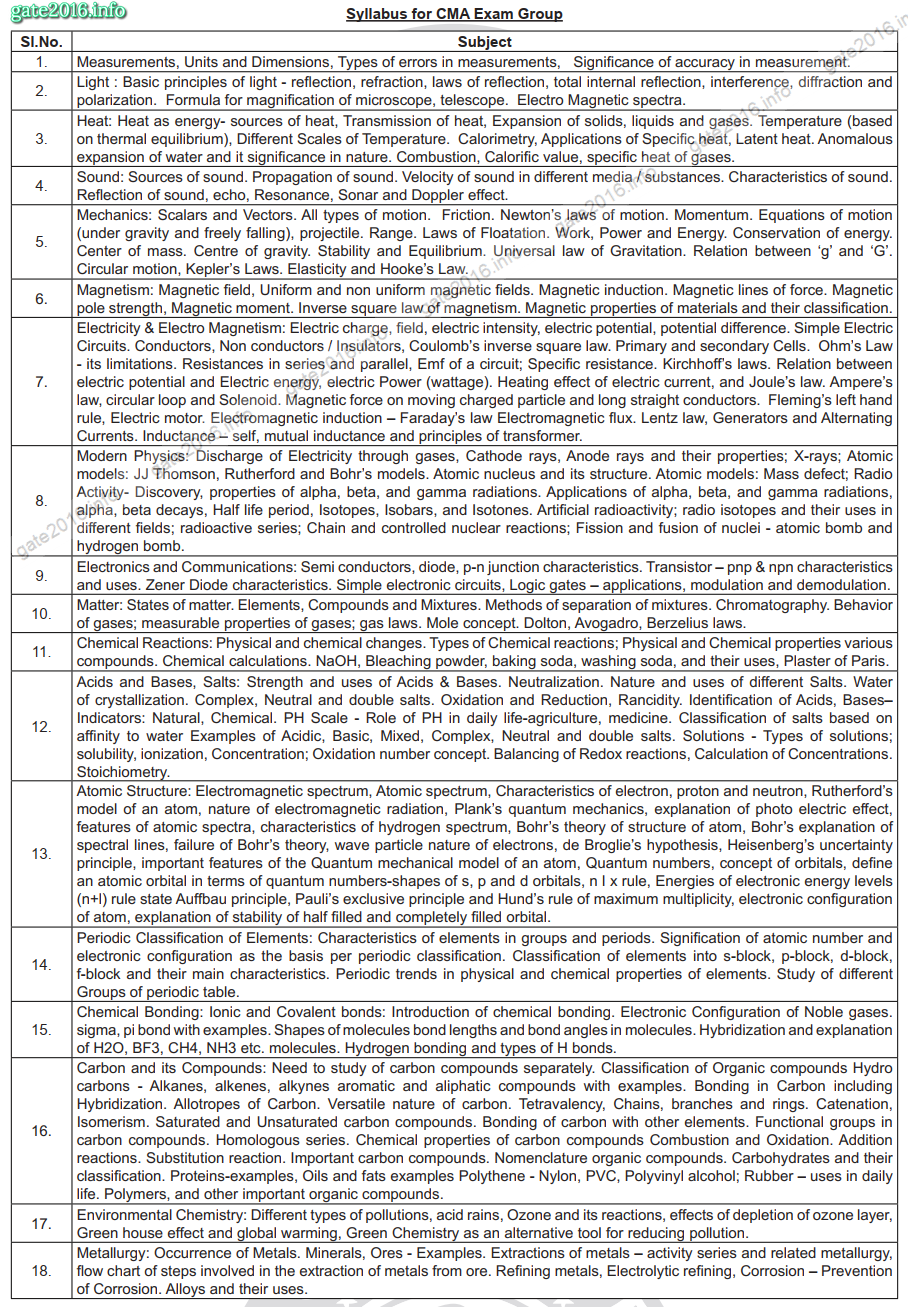
Chemical & Metallurgical Assistant – (CMA)
| S. No. | Subject |
| 1. | Measurements, Units and Dimensions, Types of errors in measurements, Significance of accuracy in measurement. |
| 2. | Light : Basic principles of light – reflection, refraction, laws of reflection, total internal reflection, interference, diffraction and polarization. Formula for magnification of microscope, telescope. Electro Magnetic spectra. |
| 3. | Heat: Heat as energy- sources of heat, Transmission of heat, Expansion of solids, liquids and gases. Temperature (based on thermal equilibrium), Different Scales of Temperature. Calorimetry, Applications of Specific heat, Latent heat. Anomalous expansion of water and it significance in nature. Combustion, Calorific value, specific heat of gases. |
| 4. | Sound: Sources of sound. Propagation of sound. Velocity of sound in different media / substances. Characteristics of sound. Reflection of sound, echo, Resonance, Sonar and Doppler effect. |
| 5. | Mechanics: Scalars and Vectors. All types of motion. Friction. Newton’s laws of motion. Momentum. Equations of motion (under gravity and freely falling), projectile. Range. Laws of Floatation. Work, Power and Energy. Conservation of energy. Center of mass. Centre of gravity. Stability and Equilibrium. Universal law of Gravitation. Relation between ‘g’ and ‘G’. Circular motion, Kepler’s Laws. Elasticity and Hooke’s Law. |
| 6. | Magnetism: Magnetic field, Uniform and non uniform magnetic fields. Magnetic induction. Magnetic lines of force. Magnetic pole strength, Magnetic moment. Inverse square law of magnetism. Magnetic properties of materials and their classification. |
| 7. | Electricity & Electro Magnetism: Electric charge, field, electric intensity, electric potential, potential difference. Simple Electric Circuits. Conductors, Non conductors / Insulators, Coulomb’s inverse square law. Primary and secondary Cells. Ohm’s Law – its limitations. Resistances in series and parallel, Emf of a circuit; Specific resistance. Kirchhoff’s laws. Relation between electric potential and Electric energy, electric Power (wattage). Heating effect of electric current, and Joule’s law. Ampere’s law, circular loop and Solenoid. Magnetic force on moving charged particle and long straight conductors. Fleming’s left hand rule, Electric motor. Electromagnetic induction – Faraday’s law Electromagnetic flux. Lentz law, Generators and Alternating Currents. Inductance – self, mutual inductance and principles of transformer. |
| 8. | Modern Physics: Discharge of Electricity through gases, Cathode rays, Anode rays and their properties; X-rays; Atomic models: JJ Thomson, Rutherford and Bohr’s models. Atomic nucleus and its structure. Atomic models: Mass defect; Radio Activity- Discovery, properties of alpha, beta, and gamma radiations. Applications of alpha, beta, and gamma radiations, alpha, beta decays, Half life period, Isotopes, Isobars, and Isotones. Artificial radioactivity; radio isotopes and their uses in different fields; radioactive series; Chain and controlled nuclear reactions; Fission and fusion of nuclei – atomic bomb and hydrogen bomb. |
| 9. | Electronics and Communications: Semi conductors, diode, p-n junction characteristics. Transistor – pnp & npn characteristics and uses. Zener Diode characteristics. Simple electronic circuits, Logic gates – applications, modulation and demodulation. |
| 10. | Matter: States of matter. Elements, Compounds and Mixtures. Methods of separation of mixtures. Chromatography. Behavior of gases; measurable properties of gases; gas laws. Mole concept. Dolton, Avogadro, Berzelius laws. |
| 11. | Chemical Reactions: Physical and chemical changes. Types of Chemical reactions; Physical and Chemical properties various compounds. Chemical calculations. NaOH, Bleaching powder, baking soda, washing soda, and their uses, Plaster of Paris. |
| 12. | Acids and Bases, Salts: Strength and uses of Acids & Bases. Neutralization. Nature and uses of different Salts. Water of crystallization. Complex, Neutral and double salts. Oxidation and Reduction, Rancidity. Identification of Acids, Bases– Indicators: Natural, Chemical. PH Scale – Role of PH in daily life-agriculture, medicine. Classification of salts based on affinity to water Examples of Acidic, Basic, Mixed, Complex, Neutral and double salts. Solutions – Types of solutions; solubility, ionization, Concentration; Oxidation number concept. Balancing of Redox reactions, Calculation of Concentrations. Stoichiometry. |
| 13. | Atomic Structure: Electromagnetic spectrum, Atomic spectrum, Characteristics of electron, proton and neutron, Rutherford’s model of an atom, nature of electromagnetic radiation, Plank’s quantum mechanics, explanation of photo electric effect, features of atomic spectra, characteristics of hydrogen spectrum, Bohr’s theory of structure of atom, Bohr’s explanation of spectral lines, failure of Bohr’s theory, wave particle nature of electrons, de Broglie’s hypothesis, Heisenberg’s uncertainty principle, important features of the Quantum mechanical model of an atom, Quantum numbers, concept of orbitals, define an atomic orbital in terms of quantum numbers-shapes of s, p and d orbitals, n l x rule, Energies of electronic energy levels (n+l) rule state Auffbau principle, Pauli’s exclusive principle and Hund’s rule of maximum multiplicity, electronic configuration of atom, explanation of stability of half filled and completely filled orbital. |
| 14. | Periodic Classification of Elements: Characteristics of elements in groups and periods. Signification of atomic number and electronic configuration as the basis per periodic classification. Classification of elements into s-block, p-block, d-block, f-block and their main characteristics. Periodic trends in physical and chemical properties of elements. Study of different Groups of periodic table. |
| 15. | Chemical Bonding: Ionic and Covalent bonds: Introduction of chemical bonding. Electronic Configuration of Noble gases. sigma, pi bond with examples. Shapes of molecules bond lengths and bond angles in molecules. Hybridization and explanation of H2O, BF3, CH4, NH3 etc. molecules. Hydrogen bonding and types of H bonds. |
| 16. | Carbon and its Compounds: Need to study of carbon compounds separately. Classification of Organic compounds Hydro carbons – Alkanes, alkenes, alkynes aromatic and aliphatic compounds with examples. Bonding in Carbon including Hybridization. Allotropes of Carbon. Versatile nature of carbon. Tetravalency, Chains, branches and rings. Catenation, Isomerism. Saturated and Unsaturated carbon compounds. Bonding of carbon with other elements. Functional groups in carbon compounds. Homologous series. Chemical properties of carbon compounds Combustion and Oxidation. Addition reactions. Substitution reaction. Important carbon compounds. Nomenclature organic compounds. Carbohydrates and their classification. Proteins-examples, Oils and fats examples Polythene – Nylon, PVC, Polyvinyl alcohol; Rubber – uses in daily life. Polymers, and other important organic compounds. |
| 17. | Environmental Chemistry: Different types of pollutions, acid rains, Ozone and its reactions, effects of depletion of ozone layer, Green house effect and global warming, Green Chemistry as an alternative tool for reducing pollution. |
| 18. | Metallurgy: Occurrence of Metals. Minerals, Ores – Examples. Extractions of metals – activity series and related metallurgy, flow chart of steps involved in the extraction of metals from ore. Refining metals, Electrolytic refining, Corrosion – Prevention of Corrosion. Alloys and their uses |
Syllabus for Chemical & Metallurgical Assistant (CMA) – JE
♣ Click Image to Enlarge.
| RRB (Railways) Technical Guide Books |
Related Links
Click below given links to get further information.